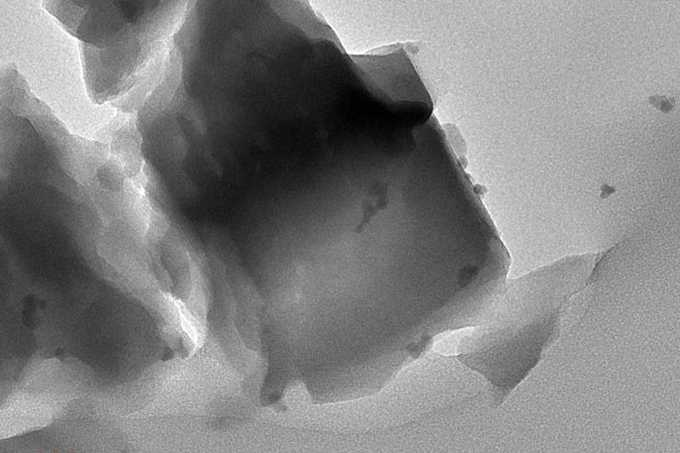
MIT researchers have developed an innovative catalyst system that converts methane—a potent greenhouse gas—into valuable polymers under ordinary conditions, offering a promising new approach to addressing climate change while creating useful materials.
Methane poses a unique challenge in the fight against climate change. Despite being less abundant than carbon dioxide, it traps significantly more heat in the atmosphere, making it a critical target for reduction. Now, a team of chemical engineers at MIT has discovered a clever way to transform this problematic gas into valuable materials using a novel two-part catalyst that works at room temperature and normal atmospheric pressure.
The research team, led by Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT, combined two unlikely partners: a common mineral called zeolite and a natural enzyme found in bacteria, fungi, and plants. This hybrid system performs a sophisticated chemical dance, converting methane first to methanol and then to formaldehyde, which can be used to create useful polymers.
What makes this approach particularly elegant is its self-sustaining nature. The enzyme, called alcohol oxidase, generates hydrogen peroxide as a byproduct, which then cycles back to help the zeolite continue converting more methane. This circular process eliminates the need for expensive additional chemicals that other methods require.
The system achieves remarkable efficiency, with methane-to-formaldehyde selectivity exceeding 90% at room temperature. The researchers demonstrated its practical potential by using the generated formaldehyde to create a polymer material at a rate exceeding 5.0 mg per gram of catalyst per hour—matching or surpassing the growth rates of many methane-consuming organisms.
The implications for real-world applications are significant. The catalyst could be suspended in water or painted onto surfaces exposed to methane gas. The team envisions applying it inside natural gas pipelines, where it could both capture leaking methane and generate polymers that could help seal cracks—addressing two environmental concerns simultaneously.
Read more: